Corrosion (rust)
The rust is generated by the corrosion of the metal. When the metal is soaked in the water, minute local anodes and local cathodes are formed on the surface of the metal due to impurities in the metal, non-uniform concentration of dissolved oxygen or uneven temperatures. On the anode part, the iron becomes a ferrous ion of the divalence and dissolves into water. And on the cathode part, hydroxide ion (OH-) is formed by electrons discharged during the anodic reaction, with oxygen and water. As a whole, iron corrodes by the reaction of Fe+H2O+ 1/2O2→Fe(OH)2.
The iron hydroxide (Fe(OH)2) is further oxidized to become red rust (Fe2O3∙nH2O), black rust (Fe3O4∙nH2O), and blue rust (FeO∙nH2O) and accumulates on the metal surface (【Fig-01】)
Corrosion promoting factors
The dissolved oxygen is indispensable for the corrosion to progress. The oxygen is easily dissolved into the water when water comes into contact with air.
In cooling towers, the oxygen dissolves into water from air rapidly, because the contact surface area of water and air is large in order to promote the evaporation. Corrosion easily progresses in cooling-tower water systems since they contain sufficiently dissolved oxygen. Therefore corrosion occurs frequently in pipes and devices in which cooling-tower water is supplied.
Dissolved oxygen is the major cause of the corrosion in the cooling water system. Corrosion does not progress unless oxygen reaches metal surfaces. 【Fig-02】 shows the relationship between the concentration of dissolved oxygen and the corrosion rate. From this figure, you can see that the corrosion rate is in proportion to the dissolved oxygen concentration.
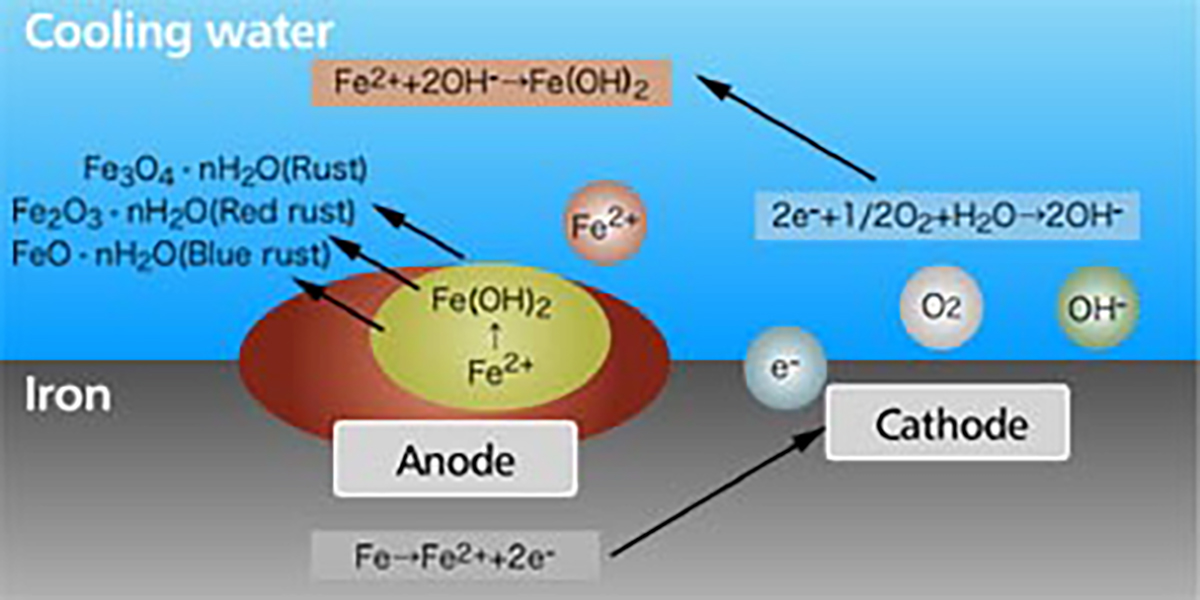
【Fig-01 Corrosion process of iron in water】
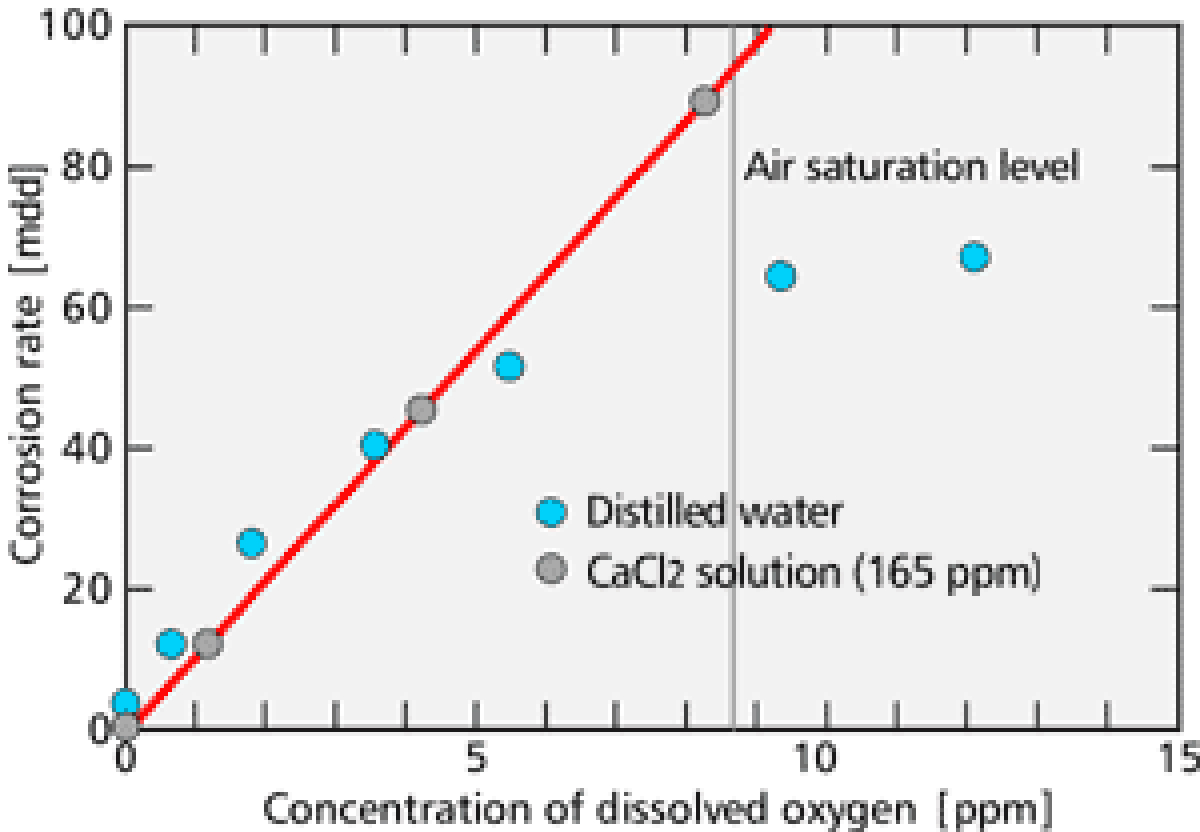
【Fig-02 Relation between oxygen concentration
and corrosion rate (25℃)】
Deoxidization anti-rusting
Corrosion can be inhibited by reducing dissolved oxygen. The amount of oxygen dissolved in the water depends on the temperature and the partial pressure of oxygen in the air contacting with the water. Cooling water usually contains dissolved oxygen with the concentration close to saturation even in a closed system. When nitrogen gas is introduced into the water, the oxygen concentration becomes lower. The reason is that nitrogen bubbles absorb dissolved oxygen so that the oxygen concentrations in water and in bubble are in an equilibrium state.
Deoxidization device O2-Free Air was developed based on this phenomenon. It separates nitrogen from compressed air using gas separation membrane and release nitrogen into water from a special nozzle as fine bubbles. The bubbles absorb dissolved oxygen while they come up to the water surface slowly.
Decrease in dissolved oxygen by deoxidization
【Fig-03】 shows three conditions: the first is the oxygen concentration of the cooling tower water, the second is the secondary cooling water without deoxidization and the third is the secondary cooling water with deoxidization. With deoxidization, dissolved oxygen in the secondary cooling water was reduced to 1/5 of that of the cooling tower water.
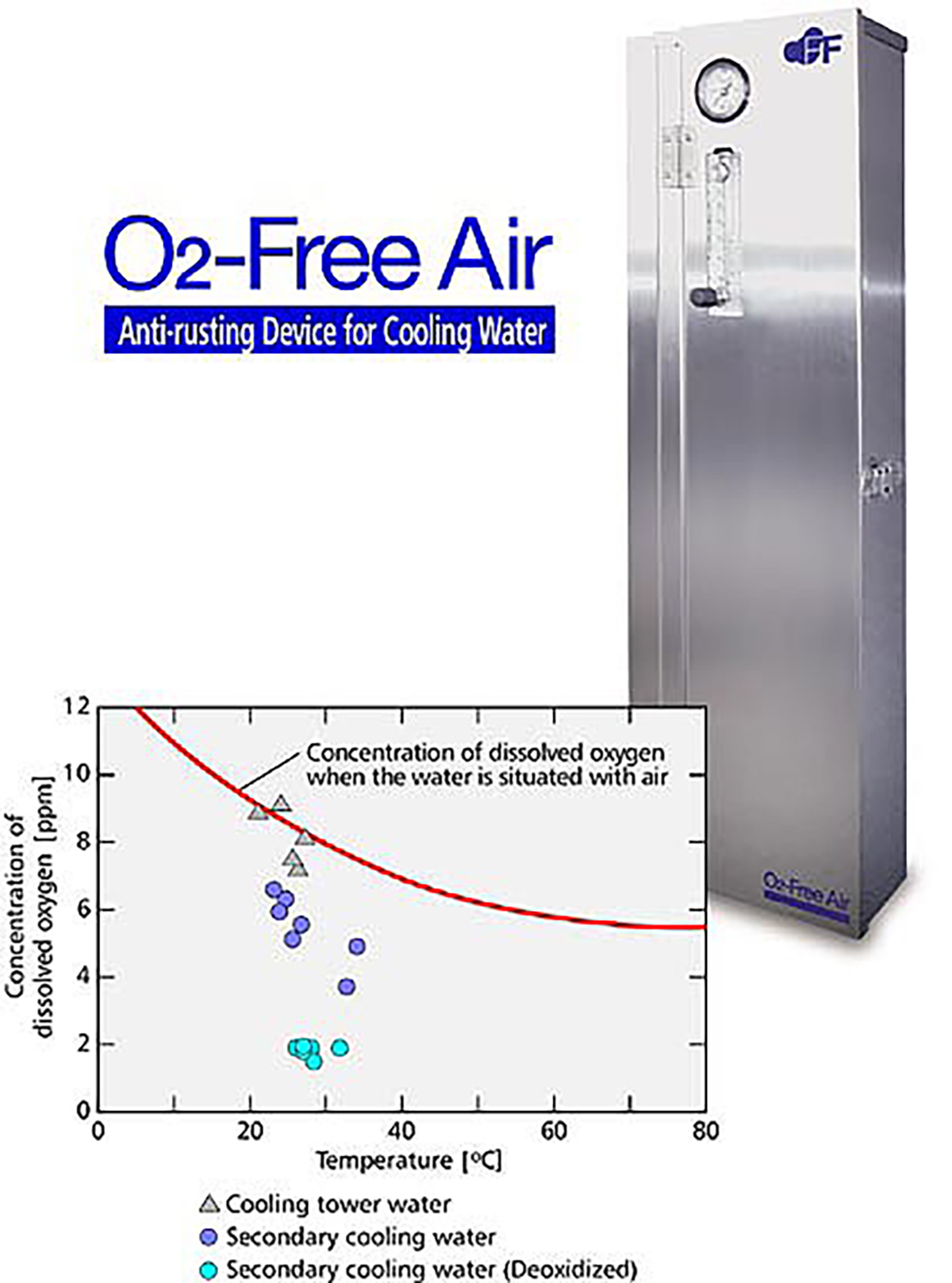
【Fig-03 Relation between temperature and oxygen concentration】
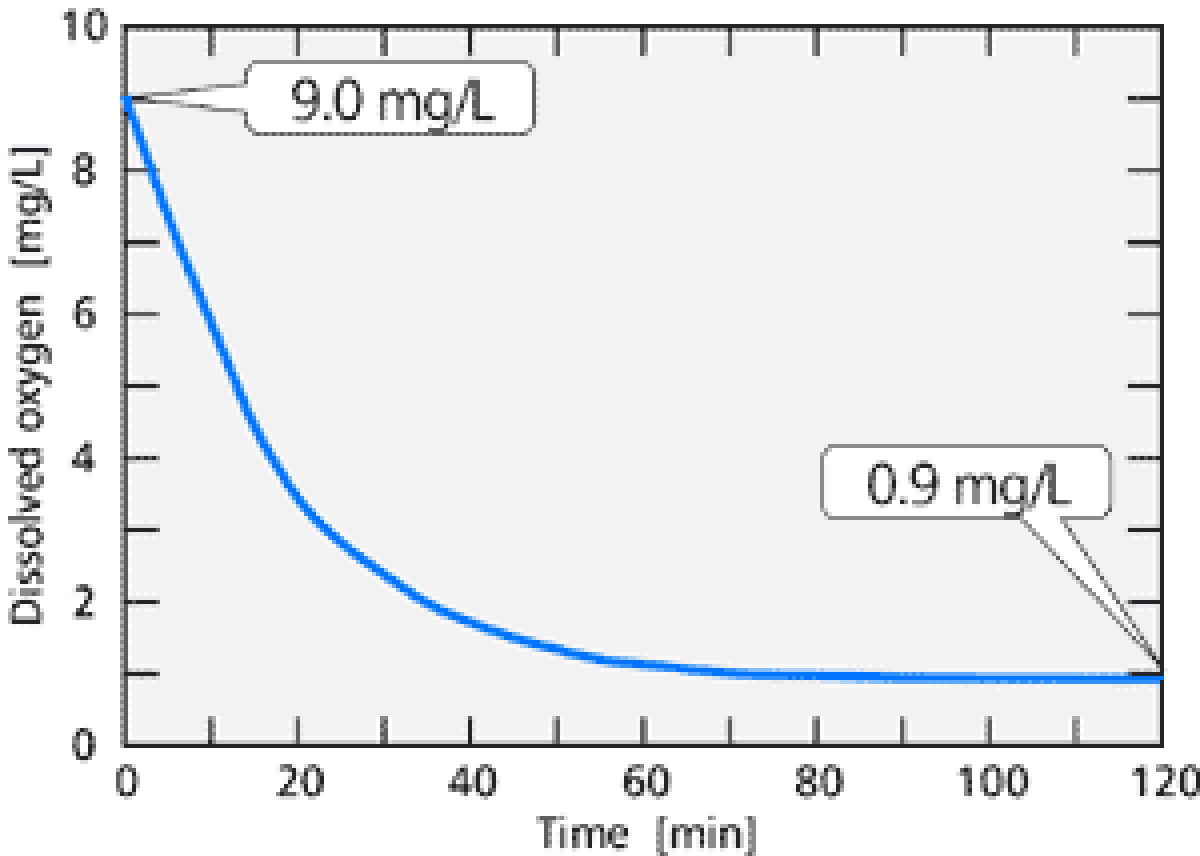
【Fig-04 Actual deoxidization】
Measured decrease in dissolved oxygen in the tank of
UWT Cooling Unit
Effect of O2-Free Air
【Fig-05】 shows a corrosion test with O2-Free Air in the right tank and without O2-Free Air in the left tank. Metal plates are hung inside the tanks, with a copper plate on the right, with iron and copper plates in the middle and an iron plate on the left.
Water in the tank without O2-Free Air turned to a bronze color showing corrosion was progressing. Actually, much rust was observed on the surface of the metal plates. On the contrary, the water in the tank with O2 Free Air was clear and metal plates inside it showed no change after 7 days.
Advantages of O2-Free Air
● Decrease in the cause of corrosion
By reducing the dissolved oxygen which is the main cause of corrosion, the corrosion rate is decreased.
● Decreased use of anti-corrosion agents
The main function of anti-corrosion agents is to prevent the contact between metal surfaces and dissolved oxygen. When the dissolved oxygen is reduced by O2-Free Air, less anti-corrosion agents are needed to prevent corrosion.
● Eco-friendly
Reduction in the use of anti-corrosion agents also results in reduction of discharged chemicals to the environment.
● Stable and highly efficient anti-corrosion effects under high temperatures
Chemicals decompose easily at high temperatures. So, anti-corrosion agents are normally added in high concentrations in high temperature operations. When dissolved oxygen is reduced by O2-Free Air, our anti-corrosion agents work well even at high temperatures. Deoxidizing corrosion prevention is best for engineering plastic molding which accompanies high mold temperatures.
Patented No.2804734
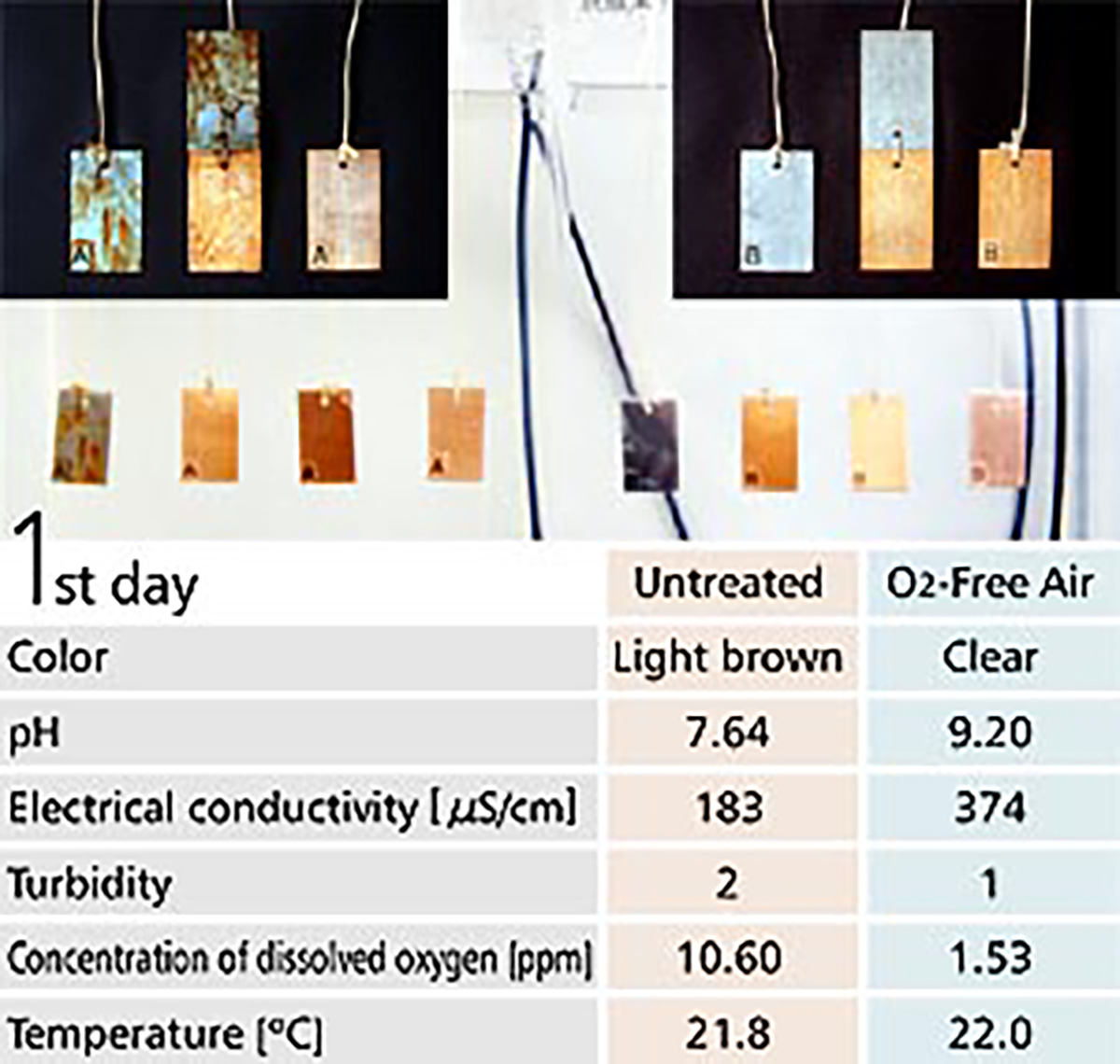
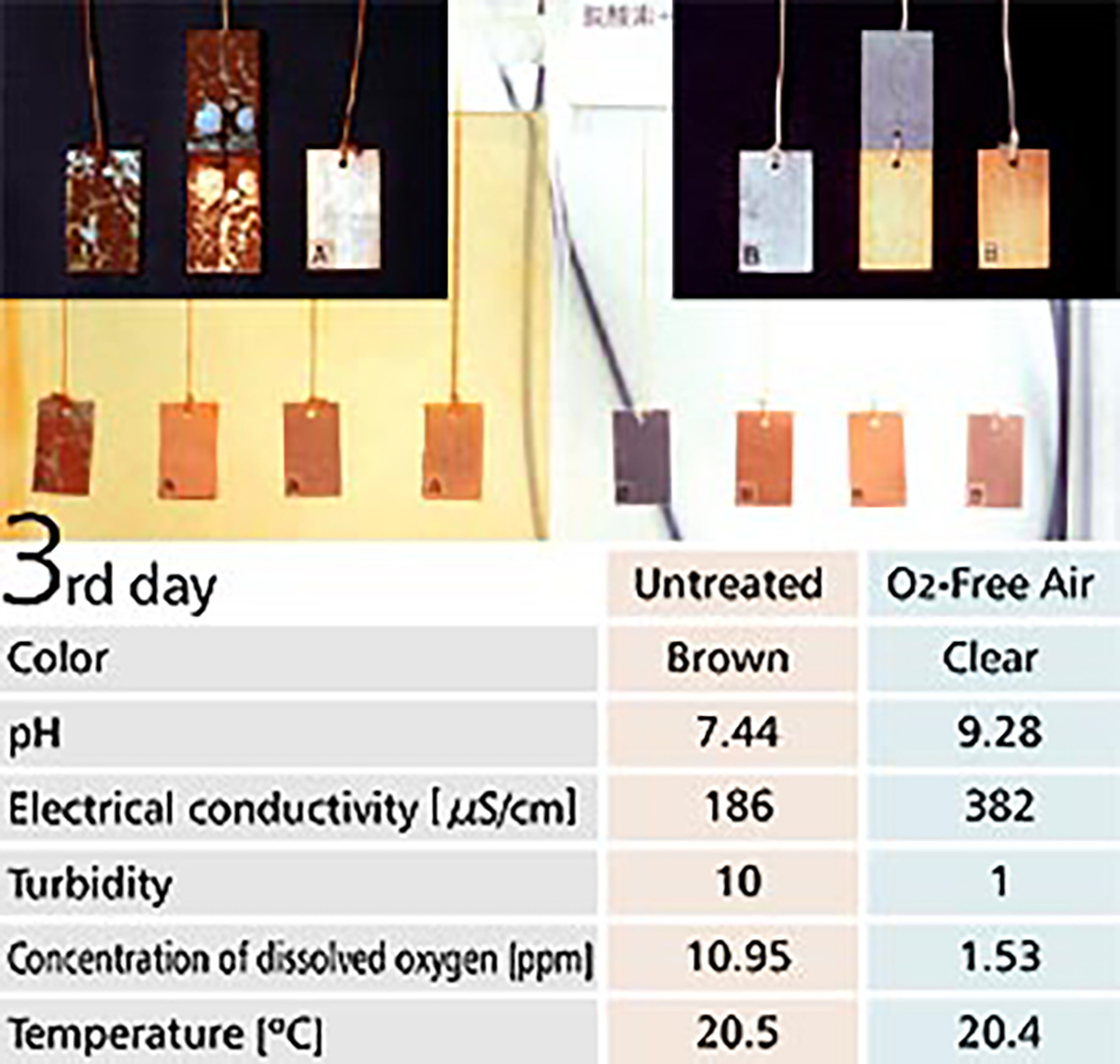
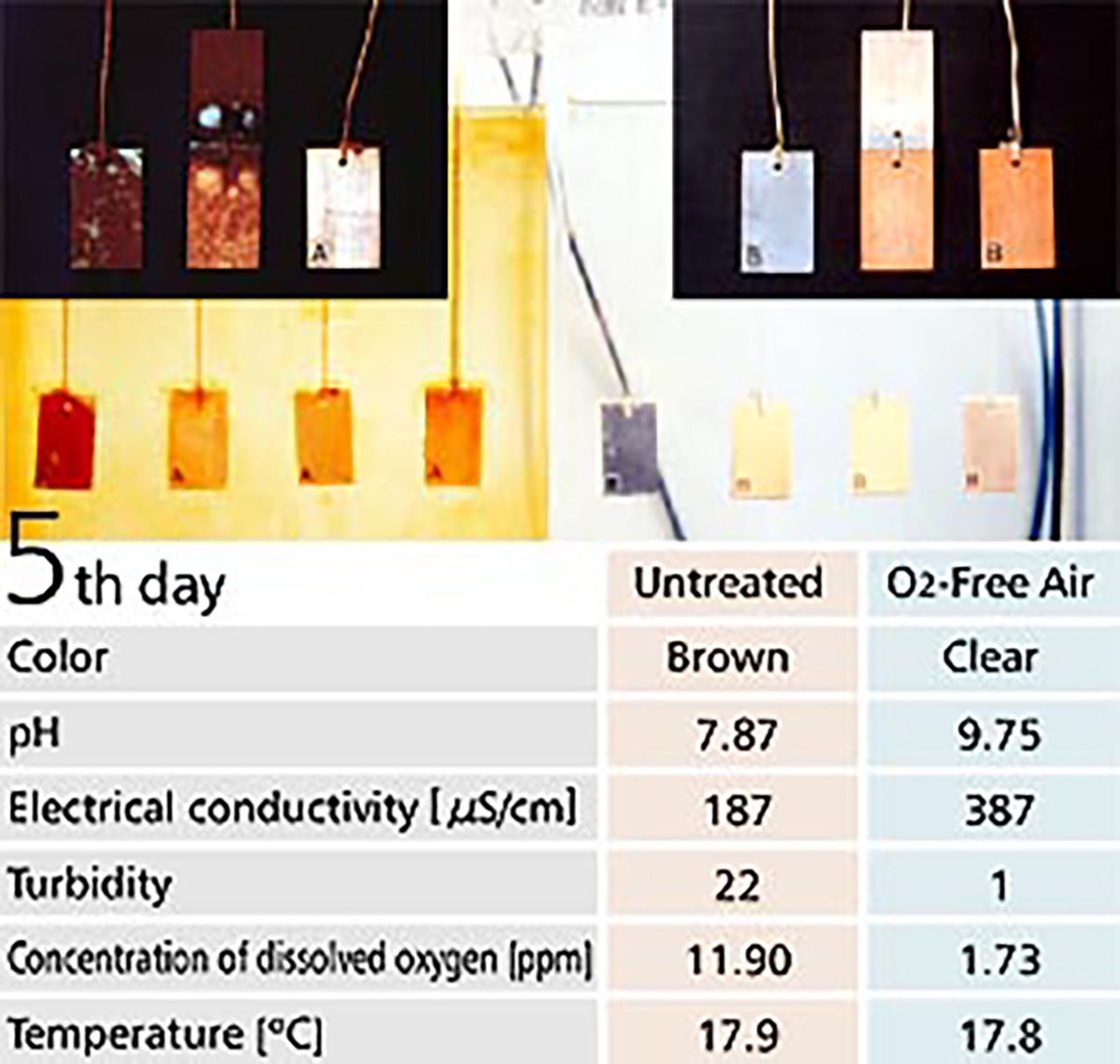
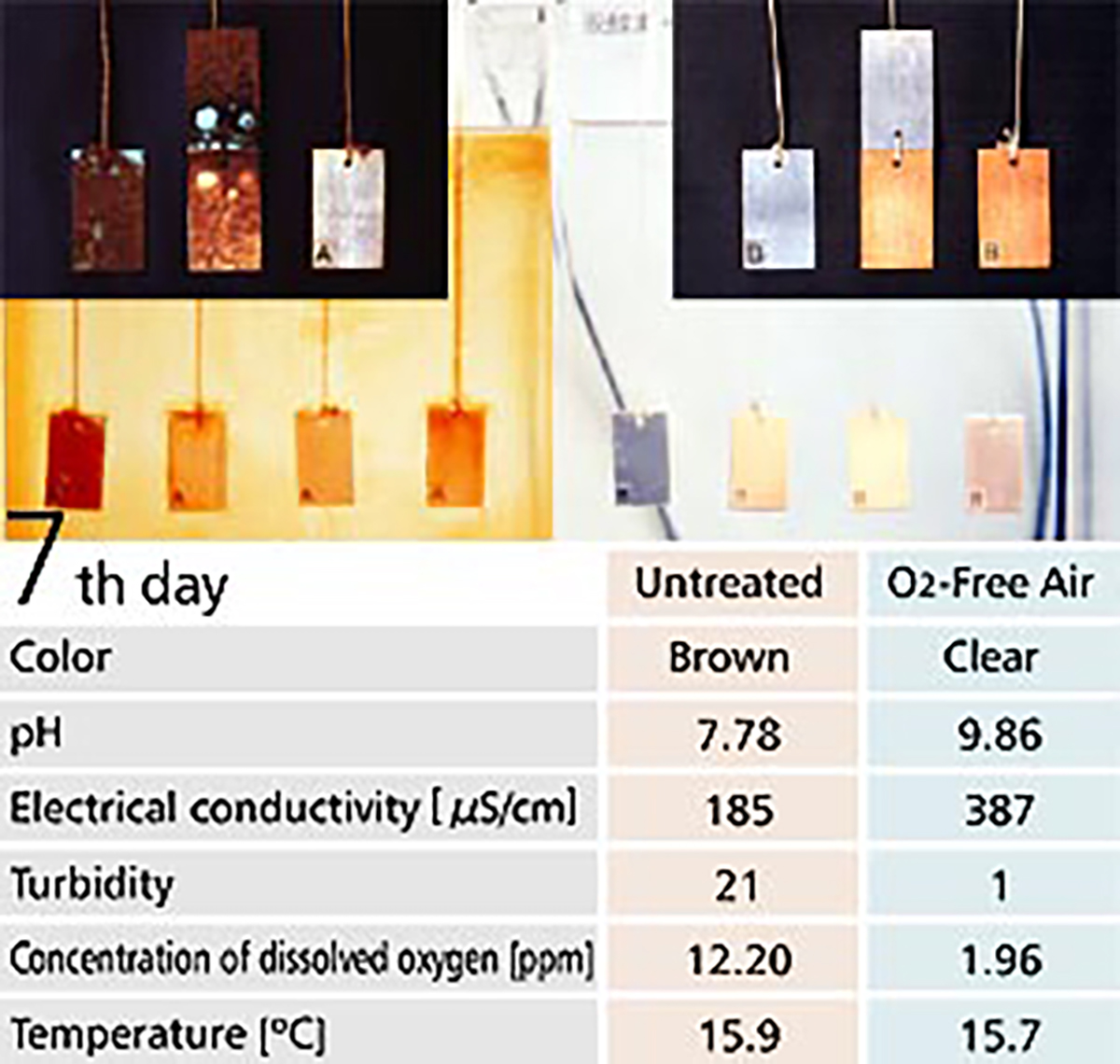
【Fig-05 Corrosion test】
Metal plates soaked in water, Untreated (left) / O2-Free Air
